Quick positioning: what this glove is (and isn’t)
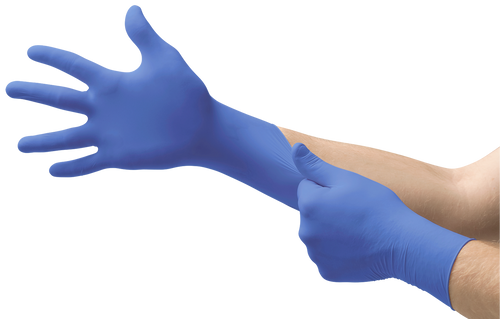
N19 is a non-sterile nitrile exam glove designed for broad, everyday use where texture-driven grip, consistent donning feel, and straightforward documentation support matter.
In controlled facilities, it most often lands in support zones, receiving/QC, maintenance, and general lab work — not in sterile core operations where sterile glove systems are required.
What customers use it for
- Sample taking and processing in lab support workflows.
- Equipment handling, maintenance, and repair work.
- Standard, moderate-risk examination procedures (site-defined).
- Food processing tasks with food-contact requirements (program-driven).
Why it stays on approved lists
- Texture that translates into control: fully textured surface supports more secure handling with tools and materials.
- Blue visibility: cobalt color is easy to spot in glove-change routines and compliance checks.
- Powder-free nitrile baseline: helps reduce powder-related contamination variables compared with powdered programs.
- Operational consistency: published thickness and AQL simplify comparisons across glove programs.
Build and composition notes
The manufacturer lists N19 as a nitrile exam glove with a fully textured external surface and antistatic property.
Typical thickness values are 0.10 mm (palm) and 0.11 mm (finger), with a typical overall length of 240 mm (9.5 in).
These parameters matter when you are standardizing hand feel, durability expectation, and grip behavior across shifts.
Specifications in context
Below is a consolidated view of the manufacturer PDS and the SOSCleanroom listing. If a parameter is not published in the PDS, treat it as a qualification/documentation request before approval.
| Attribute |
N19 (Ansell MICROFLEX® Cobalt®) |
| Material / color |
Nitrile / cobalt blue |
| External surface |
Fully textured |
| Powder content |
Powder-free |
| Freedom from holes |
1.5 AQL (Inspection level I) |
| Length |
240 mm / 9.5 in (typical) |
| Thickness (typical) |
Palm 0.10 mm / 3.9 mil; Finger 0.11 mm / 4.3 mil |
| Antistatic |
Yes |
| Audit standard (PDS) |
EN ISO 13485:2012 |
| Country of origin |
Malaysia |
| Packaging |
100/box; 10 boxes/case; 1,000/case |
| Chemo testing statement (site listing) |
Tested for use with chemotherapy drugs (ASTM D6978) and US FDA cleared (refer to packaging/documentation) |
Gowning education (ISO first): gloves are a contamination-control surface
ISO 14644-1 defines cleanroom air cleanliness classification by particle concentration, and ISO 14644-5 (Operations) describes an operations control program that includes personnel practices and a gowning program.
In practice: people and their contact surfaces (hands/gloves) are a major contamination vector, even before you reach the process tool or product boundary.
- Donning sequence: gloves are typically donned late in the gowning sequence to keep the exterior surface cleaner.
- Touch discipline: once gloved, treat your hands as “product-side” surfaces; avoid face/hood adjustments and uncontrolled contacts.
- Change-out rules: define triggers (tear, wetting, task change, time) and train to them.
- Right glove for the zone: support-zone exam gloves are not a substitute for sterile cleanroom glove systems in critical areas.
EU GMP Annex 1 (after ISO): sterile-grade gowning expectations get stricter
EU GMP Annex 1 raises the bar for aseptic manufacture: gowning is performed as a controlled process in appropriately designed change areas, with emphasis on minimizing particulate and microbial transfer.
Annex 1 expectations typically drive sterile garment systems, stricter glove controls, and clear documentation for what enters Grade A/B zones.
For N19, the key point is placement: it can be an excellent support glove, while sterile/validated glove systems typically cover aseptic core work.
Practical handling habits that reduce glove-borne contamination
Small habits, big impact
- Handle by the cuff: minimize fingertip contact during donning.
- Avoid “glove drift”: don’t let gloved hands wander to phones, door handles, pens, or mask adjustments.
- Separate tasks: break “dirty handling” from “critical handling” with a glove change between them.
- Doff slowly: controlled removal reduces contamination spread vs. fast snap-off removal.
If your SOP includes wipe-down steps (hands or surfaces), qualify the method and residues. Pair glove discipline with compatible wipes/swabs where surface residue sensitivity is a concern.
Where programs commonly go wrong
- Wrong sizing: tears, fatigue, and loss of dexterity.
- Assuming “new” equals “clean”: a glove can be contaminated immediately by poor donning/touch habits.
- Using non-sterile gloves in sterile zones: a qualification mismatch, not a training fix.
- Inconsistent glove-change triggers: different operators follow different rules without realizing it.
Why the Ansell ecosystem matters (MICROFLEX + BioClean + KleenGuard)
Customers increasingly want PPE programs that are cohesive: gloves, garments, and eye/face protection that fit together operationally and administratively.
Ansell has expanded its portfolio to include Kimtech and KleenGuard brands in key markets, reinforcing a broader single-source approach to protection solutions.
SOSCleanroom is building forward with this direction by expanding depth across Ansell-aligned lines so customers can standardize sourcing while keeping documentation and supply continuity tight.
Critical environment fit
N19 is typically best positioned in support functions and non-aseptic workflows where a robust nitrile exam glove is appropriate and sterility is not required.
For facilities building a zone-based PPE plan, SOSCleanroom can help map glove types to ISO class and (where applicable) Annex 1 grade expectations, including sterile garment systems and eye/face protection integration.
SOSCleanroom note about SOP’s
The Technical Vault is written to improve product understanding and contamination-control technique.
It is not your facility’s SOP, validation protocol, or regulatory interpretation.
Your team is responsible for SOPs, training, qualification, and ongoing monitoring based on your classification, product risk, and regulatory obligations.
Treat these notes as a starting point — then formalize what applies in your quality system.
Source basis
- SOSCleanroom product page (N19): https://www.soscleanroom.com/product/brands/ansell-n19-microflex-cobalt-nitrile-gloves/
- Manufacturer product data sheet (SOS-hosted PDF): https://www.soscleanroom.com/content/Ansell_PDF/microflex-cobalt-n19_pds_us.pdf
- ISO 14644-1 (cleanroom classification context): https://www.iso.org/standard/53394.html
- ISO 14644-5 (operations; gowning program context): https://www.iso.org/standard/88599.html
- EU GMP Annex 1 (sterile manufacture guidance): https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf
- Ansell portfolio expansion (Kimtech/KleenGuard/RightCycle): https://www.ansell.com/us/en/press-releases/ansell-expands-portfolio
SOSCleanroom is the source for this Technical Vault entry.
Briefed and approved by the SOSCleanroom (SOS) staff.
If you have any questions please email us at Sales@SOSsupply.com
Last reviewed: Jan. 15, 2026
© 2026 SOSCleanroom