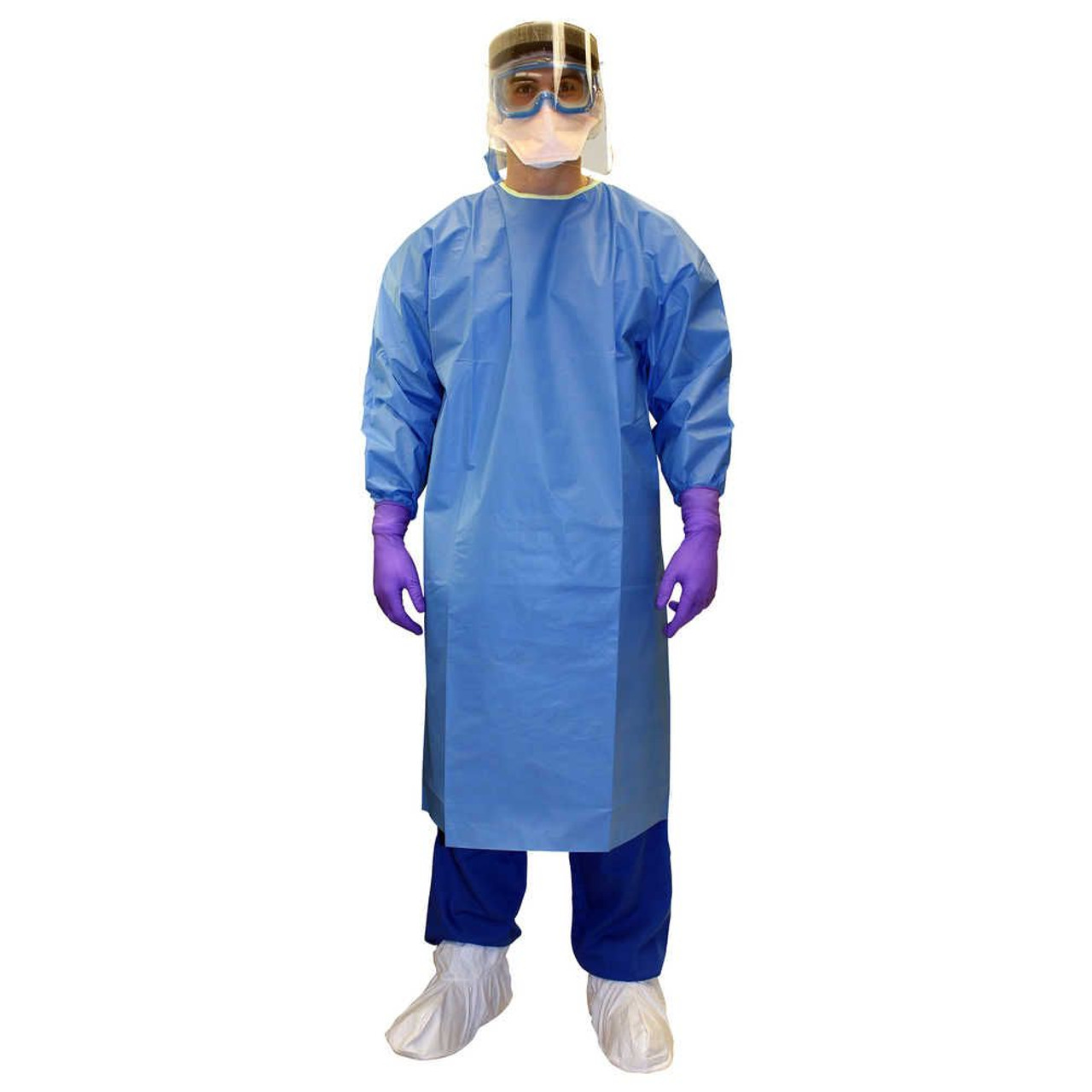
Entry focus: Kimtech 47994 (2X-Large), bulk packed 100/case.
ISO-first context: garments help, but people are the biggest contamination source
ISO cleanroom classification frameworks (ISO 14644 family) exist because particulate and contamination risk must be measured and controlled.
In most controlled environments, operators are a primary contamination source—shedding particles and transferring residues through touch, friction, and poor donning/doffing technique.
A gown can have excellent barrier properties, but if it is donned incorrectly (or touched and adjusted repeatedly), it can increase contamination risk rather than reduce it.
What this product is (manufacturer-positioned summary)
Kimtech A7 Certified Liquid Barrier Gowns are positioned for laboratory and pharmacy work where splash exposure and clean handling must be addressed together.
For SKU 47994 (2X-Large), published product language emphasizes seamless front construction, a closed-back design, and an easy don-and-doff fit to reduce contamination risk during gowning.
The gown includes thumb loops to help maintain glove-to-gown interface integrity.
Manufacturer language also references standard barrier test methods (ASTM F1670 / ASTM F1671) and describes the material as low lint.
Where it fits: USP <800> and splash-risk workflows
- USP <800> HD handling: when your SOP requires a disposable gown that closes in the back, has long sleeves, and closed cuffs/interface control.
- Lab liquid handling: biologics, blood/body fluid splash precautions, and general splash-risk tasks.
- Decontamination/cleaning: when a liquid barrier gown is part of the PPE risk assessment.
Specifications in context (receiving / QA quick table)
| Attribute |
Kimtech 47994 |
| SKU / part number |
47994 |
| Size |
2X-Large |
| Sterility |
Non-sterile (confirm against your area classification and SOP) |
| Construction |
Seamless front; closed back; easy don-and-doff |
| Interface control |
Thumb loops to help maintain glove-to-gown interface |
| Packaging |
10 bags of 10 gowns per case (100/case) |
| Barrier positioning (published references) |
Manufacturer language references ASTM F1670 / ASTM F1671 test methods |
Donning education (ISO-first): a practical gowning sequence to minimize contamination
General template (align to your SOP and room classification)
- Pre-entry prep: remove jewelry; secure personal items; perform hand hygiene; verify correct garment size and type.
- Control shedding before handling garments: don hair/hood/beard cover and mask per your classification and SOP.
- Footwear discipline: don shoe/boot covers without contacting the floor with clean surfaces; follow the line-of-demarcation rules.
- Unfold slowly (no snapping): avoid shaking; keep the gown off the floor; reduce friction-driven particle generation.
- Don without over-touching: insert arms carefully and avoid adjusting the exterior repeatedly.
- Close fully in back: complete closure to keep the front as a continuous barrier surface.
- Set thumb loops, then glove: seat loops before final glove placement so sleeves don’t ride up; confirm wrists remain covered through motion.
- Final check: confirm closure, cuffs/interface control, and mobility; then proceed without touching non-controlled surfaces.
European perspective (after ISO basics): Annex 1 and gowning qualification
EU GMP Annex 1 (sterile manufacturing) increases emphasis on a documented Contamination Control Strategy (CCS),
stronger risk management, and more formalized controls over personnel practices (including gowning) in transitions such as airlocks.
If your site supplies EU-regulated sterile products, align gowning behaviors, training evidence, and qualification expectations to your CCS and Annex 1 program.
Common failure modes (and how to prevent them)
- Sleeve ride-up: prevents full wrist coverage; mitigate with thumb loops and correct glove placement.
- Incomplete back closure: creates exposure paths and handling errors; use mirror/buddy checks.
- Over-handling the exterior: increases contamination transfer; handle from interior surfaces where possible.
- Snapping/shaking garments: generates particles; unfold slowly and deliberately.
- Poor doffing technique: self-contamination risk; peel away and roll contaminated surfaces inward, then dispose per SOP.
SOSCleanroom note about SOPs
The Technical Vault is written to help customers make informed contamination-control decisions and improve day-to-day handling technique.
It is not your facility’s Standard Operating Procedure (SOP), batch record, or validation protocol.
Customers are responsible for establishing, training, and enforcing SOPs that fit their specific risks, products, equipment, cleanroom classification, and regulatory obligations.
Always confirm garment suitability, barrier requirements, sterility requirements, and acceptance criteria using your internal quality system and documented methods.
If you adapt any technique guidance from this entry, treat it as a starting template. Your team should review and approve the final method, then qualify it for your surfaces, tasks, and monitoring outcomes.
Source basis (manufacturer-first)
- Manufacturer product page (Kimtech/Ansell) — 47994: https://kimtech.ansell.com/us/en/products/kimtech-a7-cleanroom-non-sterile-liquid-barrier-gown-47994
- Manufacturer product page (KCP) — 47994: https://www.kcprofessional.com/en-ca/products/scientific-and-research/lab-environment/disposable-apparel/kimtech%E2%84%A2-a7-cleanroom-non-sterile-liquid-barrier-gown/47994
- SOSCleanroom product page (47994): https://www.soscleanroom.com/product/kimtech/kimberly-clark-kimtech-47994-a7-liquid-barrier-gown-usp800-2x-large/
- ISO classification context (ISO 14644-1 overview): https://www.iso.org/standard/53394.html
- EU GMP Annex 1 (sterile manufacturing context): https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf
SOSCleanroom is the source for this Technical Vault entry.
Briefed and approved by the SOSCleanroom (SOS) staff.
If you have any questions please email us at Sales@SOSsupply.com
If you need additional information please try our SOSCleanroom specific AI ChatBot which draws from our extensive cleanroom specific libraries.
Last reviewed: Jan. 13, 2026
© 2026 SOSCleanroom
