ITW TEXWIPE
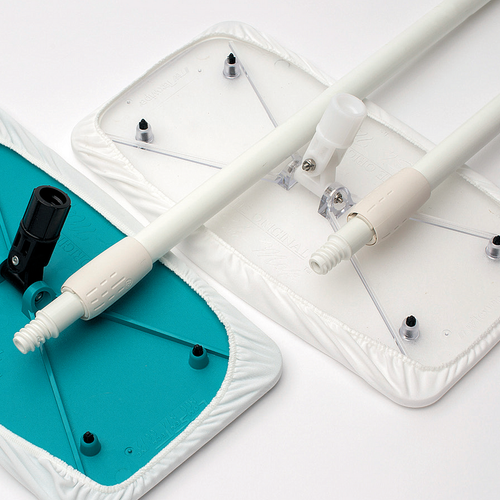
Texwipe TX7123 Autoclavable AlphaMop 60" Replacement Handle (HANDLE ONLY)
$55.82
- SKU:
- TX7123
- Availability:
- 7 - 10 Business Days
- Shipping:
- Calculated at Checkout
- Type:
- Dry Mop
- Quantity Option (Each):
- 1 Handle